Connect your patients to the support they need
Help patients make Galafold part of their routine
- The recommended dose of Galafold is 123 mg orally once every other day at the same time of day1
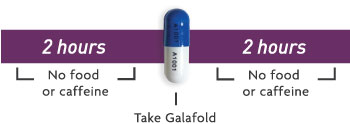
- Galafold should be taken on an empty stomach1
- Do not consume food or caffeine at least 2 hours before and 2 hours after taking Galafold to give a minimum 4 hours fast1
- The following liquids may be consumed during the 4-hour fasting period1:
-
- Water (plain, flavored, or sweetened)
- Fruit juices without pulp
- Caffeine-free carbonated beverages

INDICATIONS AND USAGE
Galafold® (migalastat) is indicated for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data.
This indication is approved under accelerated approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Help patients establish a routine
Establishing a routine for taking Galafold that fits into patients’ lifestyles may help them stay compliant with the prescribed Galafold dosing regimen.
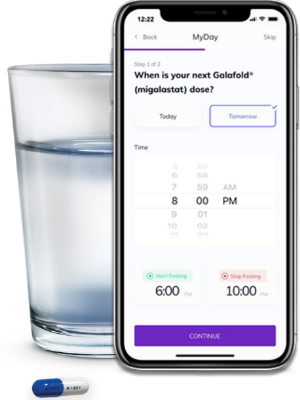
One option to help patients keep track of their Galafold routine is the Galafold® MyDay app. The app will send reminders:
- Every other day to let them know when it’s a Galafold day
- Before and after they take Galafold (to inform them when to begin and end fasting)
- When it’s time for a prescription refill, and more
Dedicated support with Amicus Assist®
Once prescribed Galafold and enrolled in the program, Amicus Assist will provide patients with a dedicated support team consisting of a Patient Education Liaison (PEL) and a Case Manager.*
What is a Patient Education Liaison?
Dedicated PELs can offer education and support to patients by:

Educating about Fabry disease and your patient’s Amicus medication

Providing tips to help patients establish a treatment routine

Helping patients have more productive conversations with their care team
PELs do not give medical advice or take the place of the patient’s healthcare provider.
What is a Case Manager?
Case Managers can help patients navigate treatment access and financial assistance by:
Helping navigate their insurance coverage

Helping to coordinate prescription delivery
Identifying possible sources of financial assistance
How can they both help?
With the combined knowledge and expertise of a PEL and a Case Manager, AMICUS ASSIST provides a two-pronged approach to ensure that your Fabry patients have access to the information and support they need.
Additional information for
patients is available
For patients looking for more information about their treatment, the Galafold patient website is available. Patients can learn more about how Galafold works, hear from other Galafold patients, and access more resources.
Patient advocacy organizations
FSIG: Fabry Support &
Information Group
National Fabry
Disease Foundation
Genetic Alliance
Rare Disease
Legislative Advocates
NORD: National Organization for Rare Disorders
Global Genes: Allies
in Rare Disease
EveryLife Foundation for Rare Diseases
Fabry International Network

Galafold® MyDay App Postcard
Download and give your patients this postcard to help them establish a routine by using the Galafold® MyDay app.
Download